Water is essential to life, yet the intricate interactions that bind H2O molecules together, known as hydrogen bonds, remain largely enigmatic. These bonds form when hydrogen and oxygen atoms within water molecules engage, sharing electronic charge in a remarkable dance. This charge-sharing forms a complex three-dimensional ‘H-bond’ network, imparting liquid water with its extraordinary characteristics.
However, the quantum phenomena underpinning these networks have only been explored through theoretical models until now.
Recently, researchers led by Sylvie Roke, head of the Laboratory for Fundamental BioPhotonics at EPFL‘s School of Engineering, have introduced an innovative method called correlated vibrational spectroscopy (CVS). This breakthrough enables researchers to observe the behaviors of water molecules within H-bond networks more precisely.
CVS uniquely distinguishes between molecules that are actively participating in these bonds and those that are randomly arranged and non-interacting. Unlike previous methods that amalgamate data from both types, CVS offers a clearer, more focused understanding of molecular interactions, paving the way for deeper insights into the nature of water itself.
“Current spectroscopy methods measure the scattering of laser light caused by the vibrations of all molecules in a system, so you have to guess or assume that what you are seeing is due to the molecular interaction you’re interested in,” Roke explains. “With CVS, the vibrational mode of each different type of molecule has its own vibrational spectrum. And because each spectrum has a unique peak corresponding to water molecules moving back and forth along the H-bonds, we can measure directly their properties, such as how much electronic charge is shared, and how H-bond strength is impacted.”
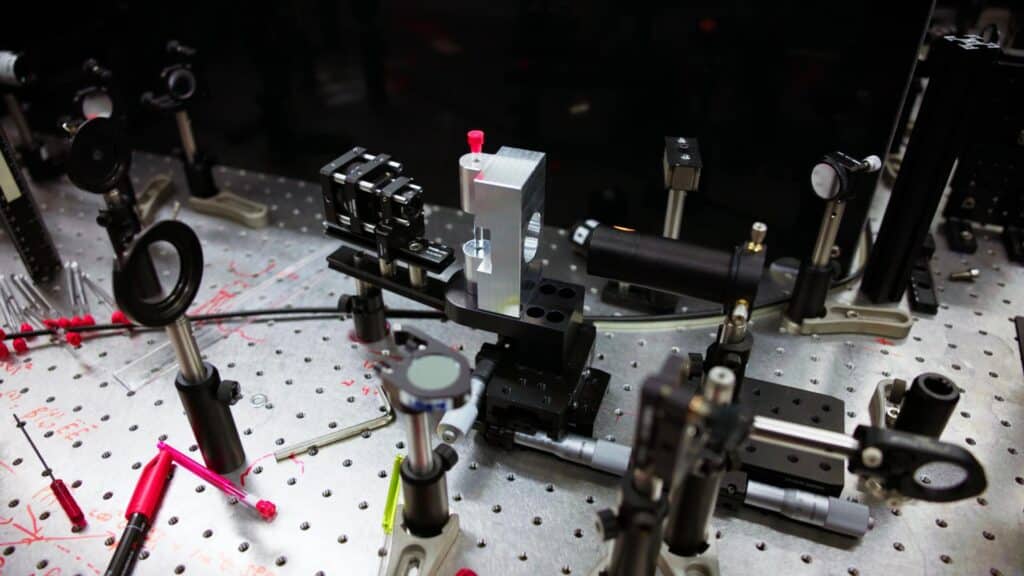
The correlated vibrational spectroscopy (CVS) setup. Credit: Jamani Caillet
The team says the method holds “transformative” potential for characterizing interactions in all types of materials. By illuminating liquid water with femtosecond (one quadrillionth of a second) laser pulses in the near-infrared spectrum, the researchers can effectively distinguish between interacting and non-interacting molecules. These ultra-short bursts generate tiny charge oscillations and atomic displacements in the water, triggering the emission of visible light.
The resulting scattering pattern reveals vital information about the molecules’ spatial arrangement, while the color of the emitted photons provides insights into atomic displacements both within and among molecules.
“Typical experiments place the spectrographic detector at a 90-degree angle to the incoming laser beam, but we realized that we could probe interacting molecules simply by changing the detector position and recording spectra using certain combinations of polarized light. In this way, we can create separate spectra for non-interacting and interacting molecules,” Roke says.
A water sample in a tube for CVS analysis.
The research team undertook a series of experiments using CVS to effectively disentangle the electronic and nuclear quantum effects within hydrogen bond networks. By altering the pH of water through the addition of hydroxide ions to increase basicity or protons to enhance acidity, they explored new dimensions of molecular interaction.
“Hydroxide ions and protons participate in H-bonding, so changing the pH of water changes its reactivity,” says PhD student Mischa Flór, the paper’s first author. “With CVS, we can now quantify exactly how much extra charge hydroxide ions donate to H-bond networks (8%), and how much charge protons accept from it (4%) – precise measurements that could never have been done experimentally before.”
Advanced simulations carried out in collaboration with experts from France, Italy, and the UK have provided insightful explanations for these values.
The researchers highlight that this innovative method, validated through rigorous theoretical calculations, is universally applicable to any material. Excitingly, several new characterization experiments are already in progress, demonstrating its broad potential.
“The ability to quantify directly H-bonding strength is a powerful method that can be used to clarify molecular-level details of any solution, for example, containing electrolytes, sugars, amino acids, DNA, or proteins,” Roke says. “As CVS is not limited to water, it can also deliver a wealth of information on other liquids, systems, and processes.”
Journal reference:
Mischa Flór, David M. Wilkins, Miguel de la Puente, Damien Laage, Giuseppe Cassone, Sylvie Roke. Dissecting the hydrogen bond network of water: Charge transfer and nuclear quantum effects. Science, 2024; DOI: 10.1126/science.ads4369